Engineered for precision and efficiency, pneumatic conveying of bulk ingredients utilizes the force of air or specialty gas to move bulk materials through intricate pipelines from delivery to storage to production.
Bulk Ingredient Handling in the Pharmaceuticals Industry
Let AZO engineer the sanitary, hygienic bulk handling system that safeguards your pharmaceuticals manufacturing processes with highly accurate, safe, cross-contamination free equipment.
Bulk Ingredient Handling for the Pharmaceuticals Industry: Overview
Manufacturing pharmaceuticals — such as prescription or over-the-counter-medications — requires handling a broad range of expensive bulk dry ingredients. Powders such as TiO2, finished mixes & blends, or diatomaceous earth are used to make the medications consumers rely on to improve their health and well-being. In this industry minimizing waste, precise measurements, and accurate recordkeeping are essential. Whether you’re manufacturing over-the-counter medications, ointments, creams, or other pharmaceutical products, trust AZO’s decades of experience designing, engineering, and supporting safe and reliable bulk material handling systems in operations where product purity is critical.

Typical Pharmaceuticals Industry System
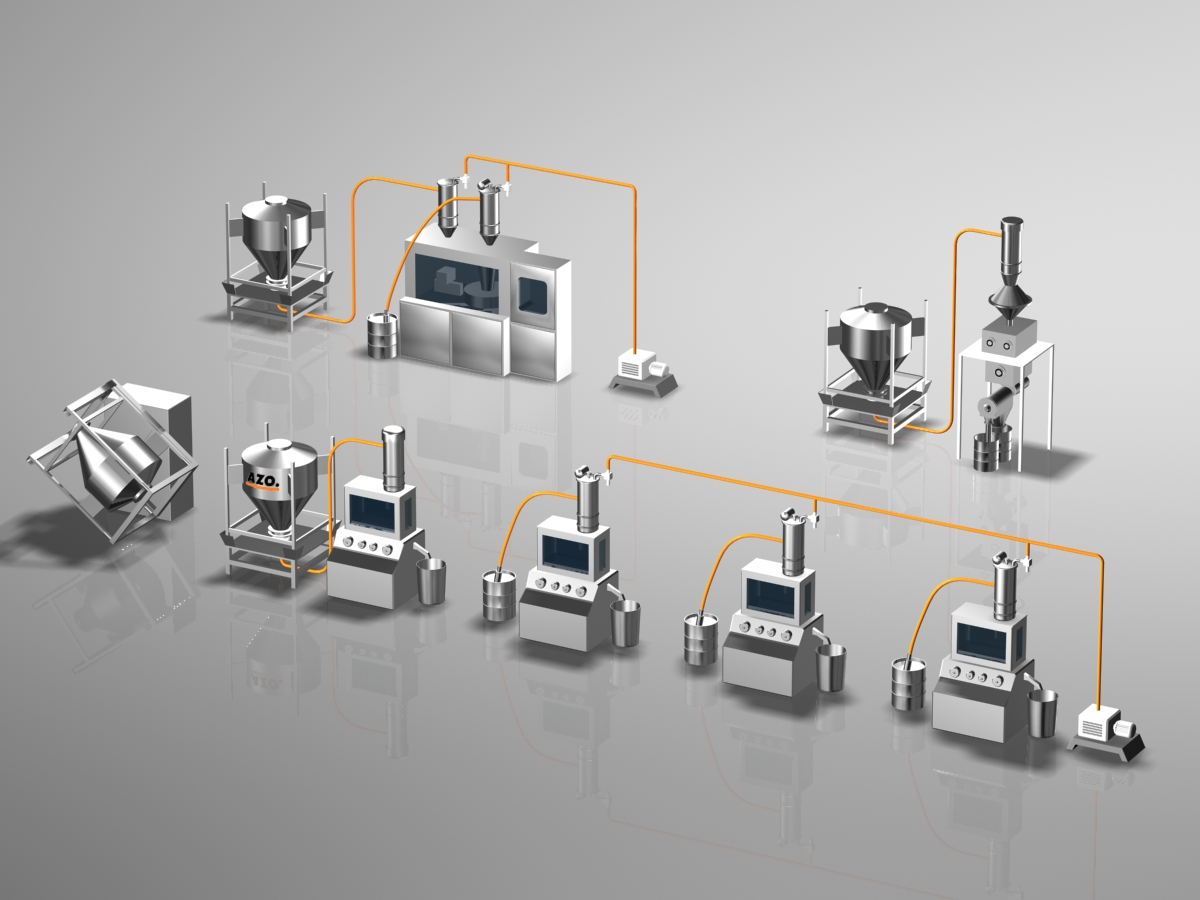
Container Tumble Blender
IBC (Intermediate Bulk Container)
Clean Air Line
AZO designs and engineers many different types of pneumatic conveying systems for bulk ingredient handling, each uniquely designed for specific projects.
Tablet Press (Not by AZO Provided by Customer)
Vacuum Pump (Buyout Equipment)
Drum for Material Storage (Buyout Equipment)
AZO offers a comprehensive range of bulk ingredient storage systems tailored to meet the distinct needs of different materials and manufacturing processes.
Vacuum Receiver
Container Docking Station
The AZO docking system allows for easy handling while preventing contamination to the environment.
AZO Centrifugal Screener E-Series
AZO screeners ensure foreign materials never have the chance to enter your production process.
Tablet Press (Not by AZO Provided by Customer)
Bulk Ingredient Handling for the Pharmaceuticals Industry: Common Challenges
Accurate weighing of minor and micro ingredients.
Accurate weighing of minor and micro ingredients.
Accuracy is of paramount importance when weighing micro and minor ingredients, which can be extremely expensive or stored and dispensed in small quantities. To reduce waste, precise dosing is critical. Pharmaceutical ingredients also have unique properties which require special design considerations, including using small hoppers with mirror-like finishes.
Handling non-free flowing ingredients.
Handling non-free flowing ingredients.
Handling non-free flowing pharmaceutical ingredients can be exceptionally difficult, with each presenting a unique challenge. It is important to perform lab testing of non-free flowing ingredients to verify proper design parameters. These parameters must be considered when designing material storage, conditioning, weighing, dosing, and dispensing for difficult-to-handle ingredients.
Pharmaceutical product manufacturers must meet hygiene and consumer safety requirements.
Pharmaceutical product manufacturers must meet hygiene and consumer safety requirements.
Ingredient integrity and safety is a critical concern for pharmaceutical product manufacturers, as contamination can not only affect end product quality, but also pose risks to consumers. Whether dosing or weighing, pharmaceutical manufacturers must comply with proper hygienic handling practices for all bulk ingredients through regular sanitation and wash-downs. They must also eliminate cross-contamination risks, verify cleanliness, and prevent ingredient cross-contact.
Pharmaceutical manufacturers must contain dust.
Pharmaceutical manufacturers must contain dust.
A method for containing the high volumes of dust generated by many ingredients used in pharmaceutical processing should be included in every bulk material handling system design. Fugitive dust can lead to any number of problems, including operator exposure, or in the worst case, combustion. Improper dust control creates an unsafe working environment for personnel and increases the risk of product contamination.
Pharmaceutical manufacturers must properly handle hazardous materials.
Pharmaceutical manufacturers must properly handle hazardous materials.
Many of the ingredients used to make pharmaceuticals can be hazardous to employees working around them. These compounds and chemicals must be safely stored, dispensed, and conveyed within an operation. Equipment must be capable of completely containing all hazardous materials to safeguard employees’ health and to minimize insurance risks.
Government regulation and accurate lot tracking.
Government regulation and accurate lot tracking.
Government requirements for safe handling and pharmaceuticals manufacturing include mandates for accurate tracking of every ingredient used in each batch. Compliance failures such as inaccurate batching or documentation errors — mistakes that commonly occur in manual handling applications — significantly increase the risk of fines or quality issues.
Bulk Ingredients for the Pharma Industry
-
TiO2
TiO2
TiO2
Bright white and used in a variety of everyday products, TiO2 is an extremely common ingredient in many bulk ingredient handling operations, including personal products, poly and plastics, chemicals, and pharmaceuticals. Characterized by fine particles that flow poorly, TiO2 is moderately abrasive and forms heavy, dense clumps. These critical attributes can make emptying this ingredient out of storage vessels or successfully conveying it challenging. AZO’s engineering team carefully considers each of these factors to ensure the design of a TiO2 handling system is safe, reliable, and dust free.
-
Mixes & Blends
Mixes & Blends
Mixes & Blends
Using mixes and blends as ingredients to produce snacks, candy confectionery, or baking mixes and blends, requires several considerations to ensure the mix remains blended through production and packaging processes. The key to their effective bulk handling is in the engineering and design of an automated bulk process solution. The system must accommodate the unique characteristics of each mix or blend to prevent it from separating into its component parts. Dry bulk handling systems must also maintain batch control, preserve hygienic conditions, and mitigate dust. Regardless of the recipe, AZO’s design and engineering team will deliver the dry bulk ingredient handling solution that safeguards product purity and consistency.
-
Diatomaceous Earth
Diatomaceous Earth
Diatomaceous Earth
A type of crystalline silica made from pulverized sedimentary rock, diatomaceous earth comes in many forms, including pellets, particles, or powder. In its powdered form, this naturally occurring ingredient has a high surface area and light density. It is an ingredient used in a range of beverage and food grade filtration applications and in many industrial applications. Whether it’s used in manufacturing of personal products, pharmaceuticals, chemicals, or beverage filtration, diatomaceous earth powder’s unique characteristics require special material handling solutions. It is prone to producing high amounts of dust, which is a carcinogen. AZO’s engineering team carefully considers each of these factors to ensure the design of a diatomaceous earth handling system is safe, reliable, and dust free.
TiO2
Bright white and used in a variety of everyday products, TiO2 is an extremely common ingredient in many bulk ingredient handling operations, including personal products, poly and plastics, chemicals, and pharmaceuticals. Characterized by fine particles that flow poorly, TiO2 is moderately abrasive and forms heavy, dense clumps. These critical attributes can make emptying this ingredient out of storage vessels or successfully conveying it challenging. AZO’s engineering team carefully considers each of these factors to ensure the design of a TiO2 handling system is safe, reliable, and dust free.
Mixes & Blends
Using mixes and blends as ingredients to produce snacks, candy confectionery, or baking mixes and blends, requires several considerations to ensure the mix remains blended through production and packaging processes. The key to their effective bulk handling is in the engineering and design of an automated bulk process solution. The system must accommodate the unique characteristics of each mix or blend to prevent it from separating into its component parts. Dry bulk handling systems must also maintain batch control, preserve hygienic conditions, and mitigate dust. Regardless of the recipe, AZO’s design and engineering team will deliver the dry bulk ingredient handling solution that safeguards product purity and consistency.
Diatomaceous Earth
A type of crystalline silica made from pulverized sedimentary rock, diatomaceous earth comes in many forms, including pellets, particles, or powder. In its powdered form, this naturally occurring ingredient has a high surface area and light density. It is an ingredient used in a range of beverage and food grade filtration applications and in many industrial applications. Whether it’s used in manufacturing of personal products, pharmaceuticals, chemicals, or beverage filtration, diatomaceous earth powder’s unique characteristics require special material handling solutions. It is prone to producing high amounts of dust, which is a carcinogen. AZO’s engineering team carefully considers each of these factors to ensure the design of a diatomaceous earth handling system is safe, reliable, and dust free.
AZO’s Bulk Ingredient Handling Solutions for the Pharmaceutical Industry: Outcomes & Benefits
With an AZO bulk ingredient handling system, your pharmaceutical manufacturing operation will:
- Ensure product consistency and quality via highly accurate automatic weighing and dosing of bulk, minor, and micro ingredients.
- Maintain proper hygiene and eliminate cross-contamination risks with easy-to-clean equipment.
- Easily accommodate a broad range of pharmaceutical product formulations and ingredients.
- Keep employees safe by reducing ergonomic injuries and accidents.
- Consistently and accurately comply with government track-and-trace requirements.


